Disclaimer: It has been over half a year since I last looked at the chemical structures for the twenty amino acids, and it is possible that some information may be incorrect. Please notify me of any mistakes and I will fix them.
I won't give the structures of the amino acids right here, since they appear all over the Internet. If you would like to see them (it would probably be helpful while reading this page), you may visit this page, although I would suggest using a textbook instead.
All chemical structures will be of the following form:
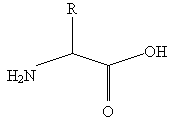
Five-membered cyclic structures should be drawn with one horizontal and two vertical sides whenever possible. Long radical chains should extend first straight upward and then travel horizontally, preferably to the right.
I will divide the amino acids into the four traditional groups based on the R groups: non-polar, polar, acidic, and basic.
Non-polar Radicals
The first seven of the nine non-polar-side groups are glycine, alanine, valine, leucine, isoleucine, methionine, and proline. Taking the first letter of each one in this order we have the nonsense word gavlimp. Generally speaking, the structures become more complex as we traverse this order. Glycine, the first of the seven, has no radical group at all (unless you count the H). Alanine has a radical of CH3. Valine has an isopropyl group; when drawn as specified above, the radical appears to be a "V".
Now, we arbitrarily associate the letter "L" with four carbons. (This seems pointless now, but it is useful later. Imagine that an L is part of a rectangle, which has 4 corners.) Leucine's radical is made up of a CH2 attached to an isopropyl. When drawing, be sure that the CH2 bends to the right, and then the isopropyl will take the shape of an "L". Isoleucine, being an isomer of leucine, also has 4 C's in the side chain, but as the name begins with an "I" and not an "L" the side chain contains no "L"-shape, but is rather a straight sec-butyl. (It sort of looks like a crooked, sideways "I"...)
The name methionine indicates that it contains a methyl and thiol, or sulfur, group (it's not really a thiol, but the important atom is there). The amino acid radical is made up of a CH2CH2 connected to an S connected to a CH3. When drawn with the radical chain bending to the right, the S will be directly over the OH.
Proline's radical is a three-carbon chain which connects at the other end to the nitrogen of the base. The cyclic structure formed is a pentagon. Both proline and pentagon start with five, if that helps.
The remaining two amino acids are phenylalanine and tryptophan. Phenylalanine should not be hard to remember, as it is an alanine with a phenyl attached to the radical. Tryptophan is much more interesting, and requires a drawing:
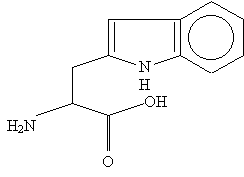
Aside from the CH2 at the beginning of the radical (of course it's there; how else could you draw the structure?) it is essentially made up of a pentagon attached by a side to a hexagon (note that the shared bond is not a triple bond nor is it of order 2.5; the benzene ring's double bonds are actually fixed). Flipping the structure upside down, the radical looks more or less like a house (the pentagon) with double walls (the double bonds), a chimney (the N), and a waterwheel (the benzene ring). More on this later.
Polar Radicals
Of the four polar-radical amino acids, two of them are the amines of carboxylic-acid-radical amino acids, namely asparagine and glutamine (from aspartic acid and glutamic acid, respectively). Asparagine's radical has one CH2 group between the base and the CONH2; glutamine has two. Because of this, asparagine, when drawn with the CH2 bending to the right, is entirely symmetrical except for the amine group in the base. In contrast, with glutamine the O of the CONH2 group ends up practically on top of the OH of the base, making the structure annoyingly difficult to draw.
The remaining two are serine and threonine. I myself can never remember these two other than the fact that they both contain OH groups. Serine is basically an alanine derivative with an OH (a lot of amino acids are alanine derivatives). Threonine's radical is a CH(OH)CH3. Notice that this means that threonine has two stereogenic centers, one at the base and one within the radical. When the Fischer projection for threonine is drawn, it has a structure not unlike the sugar threose.
Acidic Radicals
Of the four acidic radicals, you practically know two of them already! Aspartic and glutamic acids are merely asparagine and glutamine, respectively, with the amide replaced with a carboxylic acid group.
The remaining two contain no carboxyl groups. Tyrosine's structure can be remembered by its alternate name, p-hydroxyphenylalanine (it has to be para; ortho creates steric hindrance and meta doesn't provide the enhanced acidic qualities). Cysteine is the amino acid famous for bridge-bonding in hair; it is an alanine derivative with an SH group (not unlike serine). Incidentally, all of the amino acids are of the (S) stereoisomer when used for building proteins, except for cysteine, as the S atom changes the priority of the radical.
Alkaline Radicals
The three remaining amino acids all have alkaline radicals. They are lysine, arginine, and histidine. Together their initial letters spell the word lah (or hal backwards). Remember how, at the beginning, I said to associate "L" with 4? This is because lysine's radical is made up of a four-carbon chain with an NH2 at the end (to make it basic). Arginine is the molecule of threes; it has three nitrogens surrounding a central carbon; one of the nitrogens is double-bonded to the carbon and another is connected to a three-carbon chain which connects at the other end to the base. And finally we come to histidine, which has the most famous mnemonic of all, again requiring a drawing:
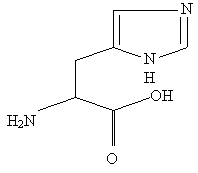
Remember the double-walled house with the waterwheel? Flip this structure over and you will see the double-walled house with a cornerstone and a chimney. Note that the cornerstone could not be on the other side of the house, for then a slight twist to the structure would make the chimney the cornerstone and the cornerstone the chimney. Note also that, in acidic solution, smoke comes out of the chimney (the other N doesn't protonate since that would cause loss of resonance).